Biomarkers, Outcome Measures, and Endpoints
by Rayna Jannes, CACNA1A Foundation Science Intern
The CACNA1A Foundation is actively engaged in an ongoing initiative to identify biomarkers, outcome measures, and endpoints for CACNA1A-related disorders. These parameters collectively serve as crucial benchmarks in clinical trials, enabling the accurate measurement of a potential treatment's impact. Without them, measuring the success of treatments in clinical trials becomes challenging, which is why it is so important to identify them. While there are currently no identified biomarkers for CACNA1A-related diseases, the CACNA1A Foundation is involved in several projects aimed at finding them, giving us hope for the future.
What exactly are biomarkers, outcome measures, and endpoints?
Biomarkers, as defined by the FDA, are characteristics that can be objectively measured as an indicator of health, disease, or a response to an intervention, such as a potential treatment. They serve as benchmarks for assessing diseases. Some biomarkers, like blood pressure or body temperature, are easy to measure. Others, such as protein levels or brain activity, require more specialized techniques like blood draws or EEGs. In clinical trials, biomarkers are often referred to as outcome measures or endpoints. They should be easy to measure and clearly correlate with a specific disorder or set of symptoms.
What are examples of biomarkers that can be used as outcome measures and endpoints?
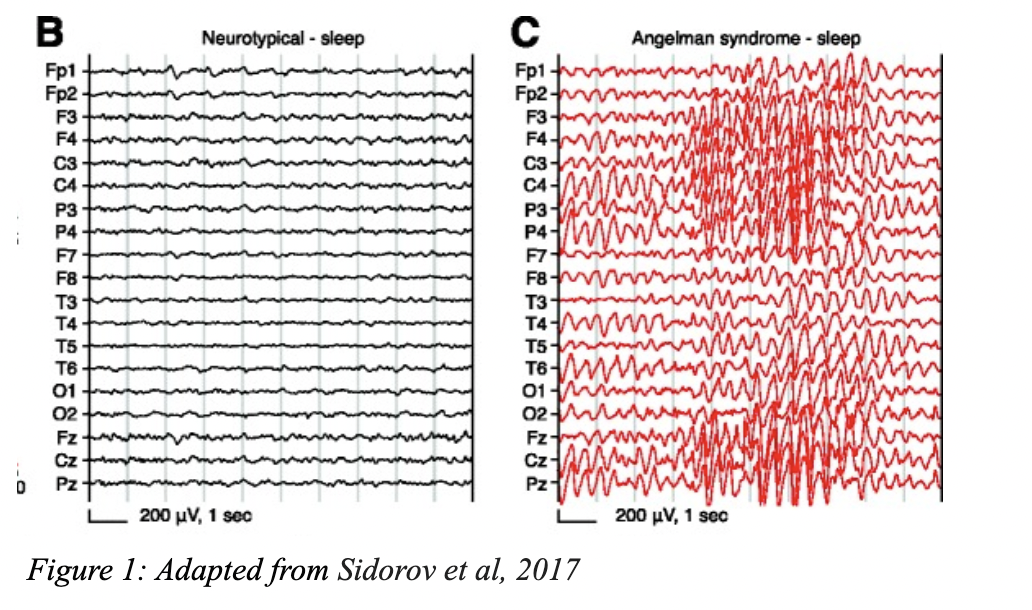
As an example, in Angelman Syndrome (AS), a rare neurodevelopmental disease characterized by seizures, ataxia, and developmental delay, delta power—a type of brain activity detected on EEG—serves as an effective biomarker. Individuals with AS exhibit significantly higher delta power compared to those without the disease (see Figure 1 below). Panel B shows the brain activity in an individual without AS. Panel C shows the brain activity in an individual with AS. These figures show a significant increase in delta power in someone living with AS (Sidorov et al., 2017).
Delta power also correlates with cognition in AS, meaning individuals with higher delta power are more severely cognitively impaired (Ostrawski et al., 2021). Because delta power is easily measured by an EEG and has a clear correlation with symptoms (e.g., cognitive impairment), it is an example of a strong biomarker that could be used as an outcome measure or endpoint to assess whether a potential treatment would work on the AS population.
How do we find biomarkers for CACNA1A-related disorders?
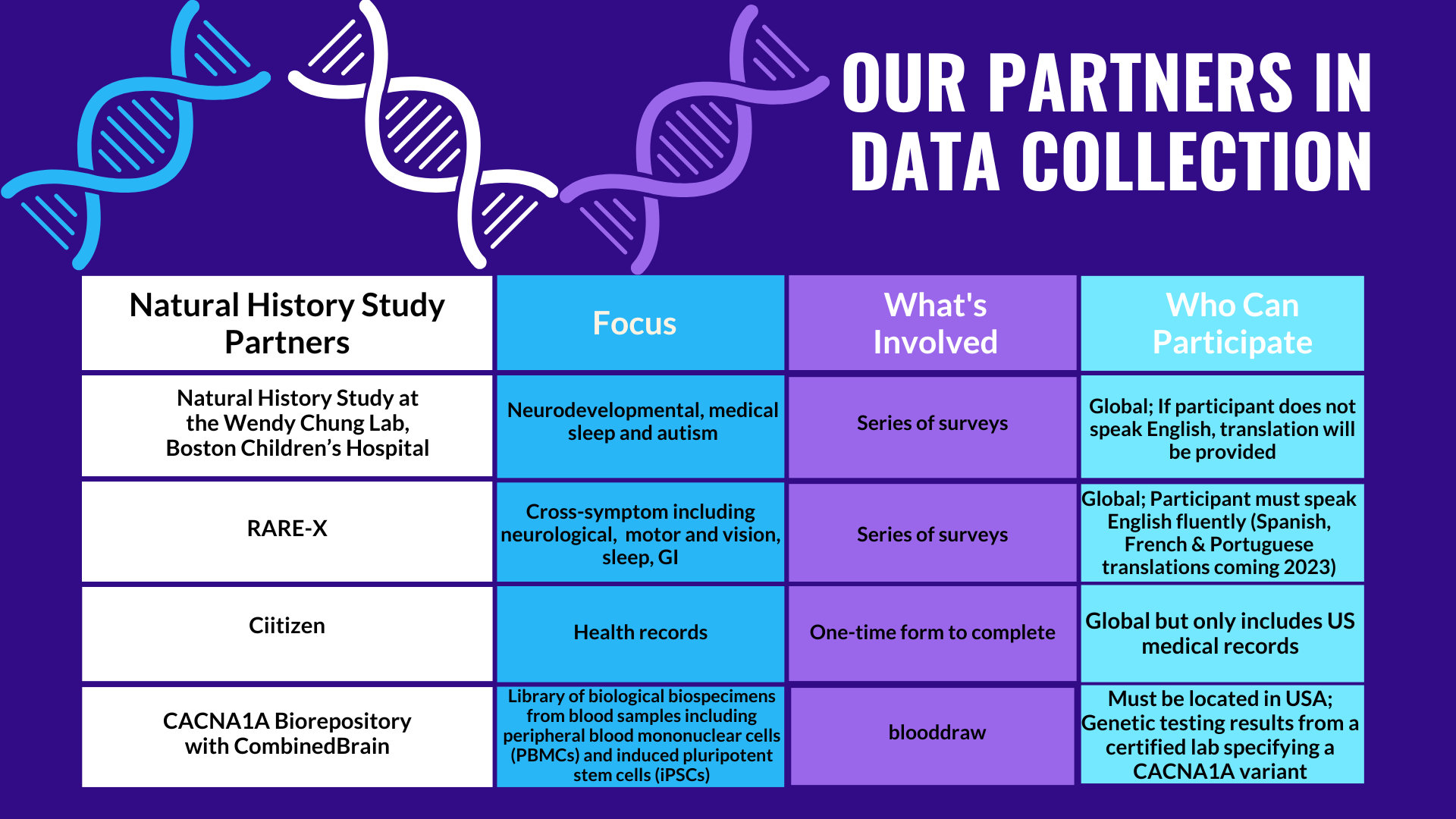
The CACNA1A Foundation is actively collaborating with patients, families, institutions, and the CACNA1A Research Network to find biomarkers akin to delta power in AS. Data from the natural history study, RARE-X, and Ciitizen offer insights into potential biomarkers, which is why large-scale participation in these studies is invaluable. By looking at when specific symptoms start/stop, how long they last, and how they change over time, clinicians and researchers can better understand the impact of a treatment. Additionally, our collaboration with COMBINEDBrain provides opportunities to search for molecular biomarkers, such as changes in proteins or compounds in the body from blood and urine samples.
At our upcoming Family Conference in Bethesda (July 19-20, 2024), neurologists who are experts in CACNA1A-related disorders such as ataxia, dystonia, eye movements, and headaches will be doing clinical assessments on our community members. This project will help us better understand biomarkers and end points for CACNA1A as clinicians can begin to validate outcome measures such as the Scale for Assessment and Rating of Ataxia (SARA) and the Observer Reported Communication Ability (ORCA) on CACNA1A patients. Future initiatives may include EEG studies or testing wearable sensors to track movement, sleep, or seizures.
In sum, identifying biomarkers, outcome measures, and endpoints hinges on the partnership of the CACNA1A community. The CACNA1A Foundation is immensely grateful to all the patients and families for their unwavering support in helping us find the best methods of testing a potential treatment and getting it approved by the FDA. Together, we are paving the way towards a better understanding and treatment of CACNA1A-related disorders.
References:
Biomarkers. U.S. Food and Drug Administration, FDA, 6 Sept. 2022, www.fda.gov/science-research/focus-areas-regulatory-science-report/focus-area-biomarkers.
Sidorov MS, Deck GM, Dolatshahi M, Thibert RL, Bird LM, Chu CJ, Philpot BD. Delta rhythmicity is a reliable EEG biomarker in Angelman syndrome: a parallel mouse and human analysis. J Neurodev Disord. 2017 May 8;9:17. doi: 10.1186/s11689-017-9195-8.
Ostrowski LM, Spencer ER, Bird LM, Thibert R, Komorowski RW, Kramer MA, Chu CJ. Delta power robustly predicts cognitive function in Angelman syndrome. Ann Clin Transl Neurol. 2021 Jul;8(7):1433-1445. doi: 10.1002/acn3.51385.